Doctoral Research
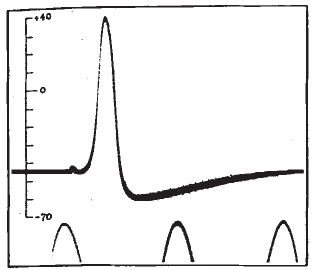
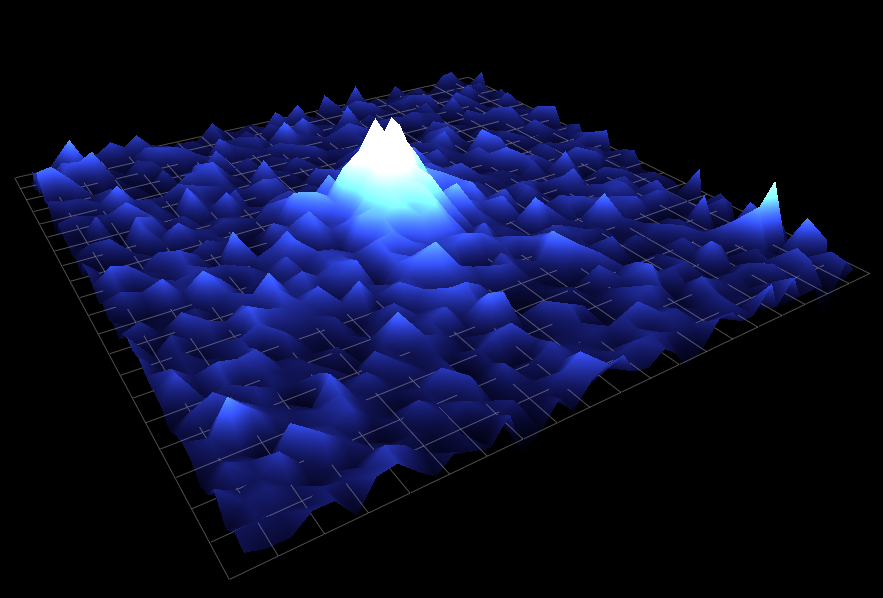
Calcium is a ubiquitous signaling molecule that regulates a large range of functions in cells, from vesicular release to homeostatic plasticity. Ca2+ can perform these diverse functions in part by acting in different spatial and temporal regimes. These range from small local transient events lasting milliseconds to cell wide elevations in cytosolic Ca2+ lasting minutes. During my time there, the main focus of the Parker lab was the IP3R, a Ca2+ channel located on the endoplasmic reticulum (ER). Upon binding both IP3 and Ca2+, the IP3R opens to release stored Ca2+ from within the ER lumen. Released Ca2+ can remain spatially restricted to a small cluster of IP3Rs to generate a local microdomain of Ca2+ (Ca2+ puff) or - depending on the proximity of neighboring IP3Rs - can propagate throughout a cell recruiting multiple puff sites through a process known as Ca2+ induced Ca2+ release.
During graduate school I worked with Dr. Ian Parker to construct a shadowless TIRF microscope and a lattice light sheet microscope, which allowed us to visualize the subcellular concentration of Ca2+ with high temporal and spatial precision. By uncaging IP3, it is possible to activate IP3Rs and measure the resulting Ca2+ puffs. I developed an algorithm which automates the detection these Ca2+ puffs and extracts their spatiotemporal parameters. I also worked on the development of another algorithm - based on techniques employed in superresolution microscopy - which uses single channel Ca2+ events to determine with nanometer precision the location of individual IP3 receptors.
Publications
Kastenschmidt J.M., Ellefsen K.L., Mannaa A.H., Giebel J.J., Yahia R., Ayer R.E., Pham P., Rios R., Vetrone S.A., Mozaffar T., Villalta S.A. QuantiMus: A Machine Learning-Based Approach for High Precision Analysis of Skeletal Muscle Morphology. Frontiers in Physiology (2019)
Ellefsen K.L., Holt J.R., Chang A.C., Nourse J.L., Arulmoli J., Mekhdjian A.H., Abuwarda H., Tombola F., Flanagan L.A., Dunn A.R., Parker I., Pathak M.M. Myosin-II mediated traction forces evoke localized Piezo1-dependent Ca2+ flickers. Communications Biology (2019)
Ellefsen K.L., Lock J.T., Settle B., Karsten C.A., Parker I. Applications of FLIKA, a Python-based image processing and analysis platform, for studying local events of cellular calcium signaling. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research (2019).
Ellefsen K.L., Parker I. Dynamic Ca2+ imaging with a simplified lattice light-sheet microscope: A sideways view of subcellular Ca2+ puffs. Cell Calcium (2018).
Lutz S.E., Smith J.R., Kim D.H., Olson C.V.L., Ellefsen K.L., Bates J.M., Gandhi S.P., Agalliu D. Caveolin1 is required for Th1 cell infiltration, but not tight junction remodeling, at the blood-brain barrier in autoimmune neuroinflammation. Cell Reports (2017).
Parker I., Evans K.T., Ellefsen K.L., Lawson D.A., Smith I.F. Lattice light sheet imaging of membrane nanotubes between human breast cancer cells in culture and in brain metastases. Scientific Reports (2017).
Figueroa D.X., Ellefsen K.L., Hathaway E.R. Yang M.C., Carathedathu M.C., Gandhi S.P Contribution of innate cortical mechanisms to the maturation of orientation selectivity in Parvalbumin interneurons. Journal of Neuroscience (2017).
Dickenson G.D., Ellefsen K.L., Dawson S.P., Pearson J.E., Parker I. Hindered cytoplasmic diffusion of inositol trisphosphate restricts its cellular range of action. Science Signaling (2016).
Ellefsen K.L., Dynes J.L., Parker I. Spinning-spot Shadowless TIRF Microscopy. PlosONE (2015).
Lock, J.T., Ellefsen K.L., Settle B., Parker I., Smith I.F. Imaging Local Ca2+ Signals in Cultured Mammalian Cells. Jove (2015).
Ellefsen K.L., Settle B., Parker I., Smith I.F. An algorithm for automated detection, localization and measurement of local calcium signals from camera-based imaging. Cell Calcium (2014).
Allison, D.W, Wilcox, B.S., Ellefsen, K.L., Askew, C.E., Hansen, D.M., Wilcox, J., Sandoval, S.S., Eggett, D., Yanagawa,Y., Steffensen, S.C. Mefloquine effects on Ventral Tegmental Area Dopamine and GABA Neuron Inhibition: a Physiologic Role for Connexin-36 Gap Junctions. Synapse (2011).